
Vol. 42, n.º 3, 2009
REVISTA
ESPAÑOLA DE
Vol. 42, n.º 3, 2009 |
CASUÍSTICA
David Parada Domínguez1, Karla Beatriz Peña González2
Servicio de Patología. Hospital Vargas. San
Francisquito a Monte Carmelo. San José, Caracas. Venezuela 1010.
1 Servicio de Patología. Hospital Vargas. Caracas. Venezuela.
2 Servicio de Patología. Instituto Oncológico «Dr. Luis Razetti».
Caracas. Venezuela.
parada@cantv.net
SUMMARY
Extrapleural solitary fibrous tumour is a mesenchymal tumour, probably of fibroblastic type, occurring only rarely in the breast. A case of solitary fibrous tumour of the breast is presented and the possible origin of this neoplasm is discussed. A 43-year-old woman presented with a ten month history of a painless, enlarging mass in the left breast, which was seen on mammography to be a 10 cm, dense, nodular, well delimited lesion,. A left mastectomy without axillary dissection was performed. Histologically, the tumour was composed of a proliferation of haphazardly distributed fusocellular elements and varying degrees of stromal collagenization. Medium-sized thin-walled blood vessels in a haemangiopericytic growth pattern were observed. The immunohistochemical study showed strong CD34 and CD99 positivity. This case represents a typical extrapleural solitary fibrous tumour with a haemangiopericytoma pattern. Our findings would indicate that the most probable origin of this lesion was the perilobular or interlobular stroma.
Keywords: Breast, solitary fibrous tumor, immunohistochemical analysis.
RESUMEN
El tumor fibroso solitario extrapleural es un tumor mesenquimal de probable origen fibroblástico. La mama es un sitio infrecuente para un tumor fibroso solitario extrapleural. Este trabajo documenta las características clínicas, histológicas e inmunohistoquímicas en un caso de tumor fibroso solitario de la mama, y discute el probable origen de esta neoplasia. Se trata de una mujer de 43 años que consultó por una masa no dolorosa en la glándula mamaria izquierda de diez meses de evolución. La mamografía izquierda mostró una lesión nodular de 10 cm densa, bien definida. Se realizó mastectomía sin vaciamiento axilar. Tras cirugía, la totalidad del tumor fue enviado para estudio histopatológico. El tumor estaba formado por una proliferación de células fusiformes distribuidas irregularmente en un estroma con áreas de variable colagenización y vasos sanguíneos de tamaño mediano y paredes delgadas en un patrón de crecimiento hemangiopericítico. El estudio inmunohistoquímico demostró fuerte positividad para CD34 y CD99. Nuestro caso representa un típico tumor fibroso solitario extrapleural con patrón hemangiopericítico. Los hallazgos descritos apoyan al estroma perilobular o interlobular como probable origen de estas lesiones.
Palabras clave: Mama, tumor fibroso solitario, inmunohistoquímica.
INTRODUCTION
Extrapleural solitary fibrous tumour (ESFT) is a mesenchymal tumour of probably fibroblastic type which shows a prominent haemangiopericytoma-like branching vascular pattern (1-3). Most extrapleural SFTs were called haemangiopericytomas in the past. The breast is an uncommon location, with a few cases documented in previous reports (4-9). This report documents the clinical, histological and immunohistochemical characteristics in a case of breast solitary fibrous tumour. We also discuss the probable origin of this neoplasm.
CASE REPORT
A 43-year-old woman presented with a painless growing mass in left mammary gland evolving for ten months. Left breast mammography showed a dense, nodular, well delimited lesion, 10 cm in diameter. Tru-cut biopsy was performed and the diagnosis was consistent with mesenchymal neoplasm, even though we could not discard fibroadenoma, phyllodes tumour, metaplastic carcinoma or pure stromal tumour. A left mastectomy without axillary dissection was performed. After surgical treatment was carried out, the whole tumour was submitted for histopathological study.
MATERIALS AND METHODS
The excised specimen was fixed in 10% buffered formalin and processed for routine histopathological study. Immunohistochemistry was performed on paraffin embedded tissue. The thin-sliced materials were immunostained by an enzyme-conjugated polymer backbone (EnVisionTM Systems, DAKO, Carpinteria, CA, USA). For specific immunohistochemical details see table 1.
RESULTS
Gross Pathology
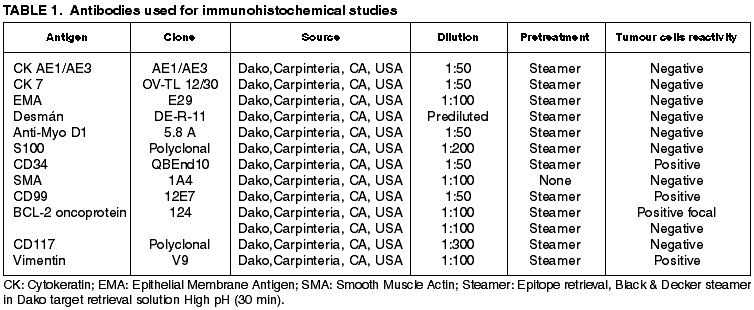
Grossly the tumour appeared as an encapsulated, tan-pink mass measuring 10 x 6 x 4 cm. At cut sections the tumour was elastic, well delimited, with gray-white areas. Neither haemorrhage nor necrosis were present. The areola-nipple complex did not show alterations (fig. 1).
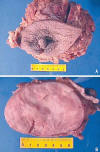
Fig. 1:
Macroscopic aspect of breast ESFT. A. Mastectomy specimen showing areola-nipple
complex without alterations. Breast skin is also normal. B. Well defined tumour,
encapsulated, no hemorrhage or necrosis present.
Histopathology
Microscopically, the tumour showed a patternless architecture characterized by a combination of hypocellular and hypercellular areas with branching, medium-sized thin-walled blood vessels in a haemangiopericytic growth pattern. The round to spindle-shaped tumour cells had little cytoplasm with indistinct borders. Nuclei were predominantly vesicular with dispersed chromatin. Cellularity greatly varied in different areas with predominance of hypercellular areas. Mitotic index was 2-3 per 10 HPF. At the periphery of the tumour a clear capsule was present. The epithelial elements were entrapped by the mesenchymal neoplastic proliferation (fig. 2).
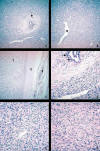
Fig. 2:
Microscopic finding. A. Typical histopathologic aspect of ESFT showing
hypocellular (peri.ductular, [arrow]) and hypercellular areas (H-E, 100X). B.
Characteristic branching haemangiopericytoma-like vessels (asterisk) (H-E,
100X). C. Well demarcated, encapsulated (C) tumour (T), normal breast tissue is
seen (arrows) (H-E, 100X). D. Detail of peri-ductular tissue, showing
hypocellular area and normal secretory epithelium (arrows) (H-E, 400X). E,F.
Characteristic spindle cell tumour cells with indistinct borders and disperse
chromatin within vesicular nuclei. No cytologic atypia or necrosis was seen
(H-E, 400X).
Immunohistochemistry
tumour cells showed expression for vimentin, CD34, CD99 (fig. 3). Focal positivity to bcl-2 was present. Epithelial, neural, muscular markers and c-kit (fig. 3) were completely negative.

Fig. 3:
Immnuhistochemical profile. A. Cytokeratin 7 stain showing a strong reactivity
in the normal ductular cells. No positivity is seen in neoplastic cells (DAB,
100X). B,C. CD34 and CD99 expression in the spindle-shaped tumour cells (DAB,
400X). D. Mast cells are inmunoreactive to CD117. Note the negativity in the
tumour cells (DAB, 400X).
DISCUSSION
Extrapleural solitary fibrous tumour is an infrequent breast neoplasm of which at least twenty cases, previously called haemangiopericytoma, were described in English literature (4-9). Clinically, patients present painless tumours, and mammograms show well circumscribed dense areas (8). Our case represents a typical ESFT example with classical branching haemangiopericytoma-like vessel pattern. We prefer the term solitary fibrous tumour rather than haemangiopericytoma since the identification of an haemangiopericytoma as a separate entity may become obsolete because its histhological features are shared by a variety of soft tissue tumours (10).
In small biopsies diagnosis represents a challenge and differential diagnoses should include cellular fibroadenoma, phyllodes tumour and metaplastic carcinoma. Cellular fibroadenoma shows proliferative stromal areas pushing out into a prominent frond-like pattern. Fibroblast density is increased, but nuclei are not enlarged or anaplastic. Some typical mitoses can be seen (11) Phyllodes tumours are macroscopically characterized by cysts. Microscopically, phylllodes tumours show frond-like proliferation of epithelial and stromal elements, and marked cellular fibroblastic components with variable mitosis and anaplasia (11) degrees. Finally, metaplastic carcinomas may be indistinguishable from mesenchymal tumours, and multiple histological sections and antibodies to keratins are usually useful in resolving this problem. The ESFT is a pure mesenchymal lesion with no frond-like epithelial proliferation of epithelial, and as we demonstrated, normal breast epithelial component is entrapped by the stromal proliferation. The use of immunohistochemichal studies is of limited value, because fibroadenomas and phyllodes tumours show similar expression for CD34, bcl-2 and CD99 (12).
The origin of breast ESFT is unknown. In normal breast, CD34 staining is seen in the perilobular and to a lesser extent in the interlobular stroma (13-16). In several tissues, CD34 positive fibroblast-like cells have been described in the mesenchyme, especially surrounding structures such as vessels and nerves in several tissues (17-20, 15-17). A number of CD34 positive lesions, such as dermatofibrosarcoma protuberans in the skin (21), intestinal fibroid polyps (22), occur at sites where there are normal CD34-positive fibroblast-like cells. Thus it has been suggested that such lesions may arise from these CD34 positive fibroblast-like cells. Interestingly, previous reports of breast ESFT, as in our case, have showed strong CD34 expression. Probably this finding is consistent with the spindle cell component of these lesions arising from the perilobular or interlobular stroma in a simmilar manner to that proposed for CD34 positive lesions at other locations.
In conclusion, we present a typical breast ESFT case. These tumours have a benign clinical course. Wide local surgery is often enough for complete tumour excision. Probably, the histogenetic origin of breast ESFT is the peri-ductular or peri-lobular CD34-fibroblast positive cell.
ACKNOWLEDGEMENTS
We thank Mrs. María Eugenia Gallinoto for technical support and assistance.
REFERENCES
Goodlad JR, Fletcher CD. Solitary fibrous tumour arising at unusual sites: analysis of a series. Histopathology 1991; 19: 515-22.
Hanau CA, Miettinen M. Solitary fibrous tumor: histological and immunohistochemical spectrum of benign and malignant variants presenting at different sites. Hum Pathol 1995; 26: 440-9.
Fukunaga M, Naganuma H, Nikaido T, Harada T, Ushigome S. Extrapleural solitary fibrous tumor: a report of seven cases. Mod Pathol 1997; 10: 443-50.
Callery CD, Rosen PP, Kinne DW. Sarcoma of the breast. A study of 32 patients with reappraisal of classification and therapy. Ann Surg 1985; 201: 527-32.
Donnell RM, Rosen PP, Lieberman PH, Kaufman RJ, Kay S, Braun DW Jr, et al. Angiosarcoma and other vascular tumors of the breast. Am J Surg Pathol 1981; 5: 629-42.
Kaufman SL, Stout AP. Hemangiopericytoma in children. Cancer 1960; 13: 695-710.
Mittal KR, Gerald W, True LD. Hemangiopericytoma of breast: report of a case with ultrastructural and immunohistochemical findings. Hum Pathol 1986; 17: 1181-3.
Tavassoli FA, Weiss S. Hemangiopericytoma of the breast. Am J Surg Pathol 1981; 5: 745-52.
Volmer J, Pickartz H, Jautzke G. Vascular tumors in the region of the breast. Virchows Arch A Pathol Anat Histol 1980; 385: 201-14.
Guillou L, Fletcher JA, Fletcher CDM, Mandani N. Extraoleural solitary fibrous tumour and haemangiopericytoma. In: Fletcher CDM, Unni K, Mertens F, editors. World Health Organization of Tumours. Pathology & Genetics. Tumours of Soft Tissue and Bone. Lyon: IARC Press, 2002.
Carter D. Stromal Lesions. In: Epstein JI editor. Interpretation of Breast Biopsies. Philadelphia: Lippincott Williams and Wilkins, 2002.
Moore T, Lee AHS. Expression of CD34 and bcl-2 in phyllodes tumours, fibroadenomas and spindle cell lesions of the breast. Histopathology 2001; 38: 62-7.
Yamazaki K, Eyden BP. Ultrastructural and immunohistochemical observations on intralobular fibroblasts of human breast, with observations on the CD34 antigen. J. Submicrosc Cyto. Pathol 1995; 27; 309-23.
Lee AHS, Happerfield LC, Bobrow LG. Comparison of four endothelial markers for assessing angiogenesis in carcinoma of the breast. J. Cellul. Pathol. 1997; 2; 67-73.
Chauhan H, Abraham A, Phillips JRA, Pringle JH, Walker RA, Jones JL. There is more than one kind of myofibroblast: analysis of CD34 expression in benign, in situ, and invasive breast lesions. J Clin Pathol 2003; 56: 271-6.
Barth PJ, Ramaswamy SE, Moll R. CD34+ fibrocytes in invasive ductal carcinoma, ductal carcinoma in situ, and benign breast lesions. Virchows Arch 2002; 440: 298-303.
Nickoloff BJ. The human progenitor cell antigen (CD34) is localised on endothelial cells, dermal dendritic cells, and perifollicular cells in formalin-fixed normal skin, and on proliferating endothelial cells and stromal spindle-shaped cells in Kaposi’s sarcoma. Arch Dermatol 1991; 127: 523-9.
Monihan JM, Carr NJ, Sobin LH. CD34 expression in stromal tumours of the gastrointestinal tract and in mesenteric fibromatoses. Histopathology 1994; 25: 469-73.
Lindenmayer AE, Miettinen M. Immunophenotypic features of uterine stromal cells. CD34 expression in endocervical stroma. Virchows Arch 1995; 426: 457-60.
Aiba S, Tabata N, Ishii H, Ootani H, Tagami H. Dermatofibrosarcoma protuberans is a unique fibrohistiocytic tumour expressing CD34. Br J Dermatol 1992; 127: 79-84.
Wille P, Borchard F. Fibroid polyps of intestinal tract are inflammatory-reactive proliferations of CD34-positive perivascular cells. Histopathology 1998; 32; 498-502.