
Vol. 41, n.º 1, 2008
REVISTA
ESPAÑOLA DE
Vol. 41, n.º 1, 2008 |
ORIGINALES
Elba Rosa Leyva-Huerta1, Constantino Ledesma-Montes1, Eunice Ortiz-Razo2, Rubén Alejandro Domínguez-Jameson3, Yolanda Torres-López2
1 Oral Pathology Laboratory. Facultad
de Odontología. Sub-Jefatura de Investigación. División de Estudios de Posgrado
e Investigación. Universidad Nacional Autónoma de México. México, D.F. México.
2 Programa Jóvenes hacia la Investigación. Facultad de Odontología.
UNAM.
3 Private Practice in Dentistry. México, D.F. México.
cotita@avantel.net /
cledezma@servidor.unam.mx
RESUMEN
Antecedentes: Hasta la fecha no existen estudios sobre el número de organizadores nucleolares como indicadores de proliferación celular en células de descamación en fumadores periodontalmente sanos y en no fumadores. Métodos: Diseñamos una modificación a la técnica argéntica de Ploton para organizadores nucleolares aplicada en 84 frotis citológicos de pacientes fumadores con y sin enfermedad periodontal. Resultados: Encontramos que el tamaño de los organizadores nucleolares fue diferente en ambos grupos. Su número varió entre 1 y 2 en las células epiteliales de los no fumadores. En los fumadores varió entre 3 y 11 organizadores nucleolares en células de la mucosa bucal y de 2 a 8 en células linguales. Conclusiones: Nuestros resultados demuestran que en los fumadores periodontalmente sanos existen un número mayor de organizadores nucleolares comparado con aquellos no fumadores. Estos resultados sugieren que a pesar de la excelente limpieza bucal realizada por los fumadores con enfermedad periodontal, ellos tienen un mayor riesgo de desarrollar lesiones pre-malignas ó aún cáncer bucal, comparados con los no fumadores sin enfermedad periodontal. Asimismo, se discuten las ventajas de nuestra modificación a la técnica que se empleó.
Palabras clave: AgNORs, regiones de organizadores nucleolares, tabaco, citología exfoliativa, enfermedad periodontal.
SUMMARY
Introduction: To date, there are no reports on the number of nucleolar organizer regions as indicators of cellular proliferation in desquamative cells from periodontally health chronic smokers and non-smokers. Methods: We designed a modification to the Ploton’s silver staining technique for nucleolar organizer regions and 84 smears from periodontally health smokers and non-smokers. Results: In this study nucleolar organizer regions’ size was different and number varied among 1 and 2 in epithelial cells from non-smokers. In smokers ranged between 3 and 11 nucleolar organizer regions in buccal mucosa cells and from 2 to 8 in tongue cells. Conclusions: Our results show that higher nucleolar organizer region number is found in periodontally health chronic smokers compared with periodontally health non-smokers. These results suggest that despite the excellent oral health made by periodontally health chronic smokers, they have a higher risk to develop pre-malignant lesions or even oral cancer, compared with periodontally health non-smokers. It is also stressed that tobacco smokers are at major risk to develop premalignant oral lesions and even oral cancer compared with non-smokers. Advantages of our modified procedure employed in this study are discussed.
Key words: AgNORs, nucleolar organizer regions, tobacco, exfoliative cytology, periodontal disease.
INTRODUCTION
Alcohol and tobacco are the main predisposing factors associated to oral cancer. The risk to suffer oral cancer is 6 times higher in smokers than in non-smokers and it is known that combined use of tobacco and alcohol exerts a higher effect (1).
Nucleolar organizer regions (NORs) are fragments of ribosomal RNA located in the nucleolus. NORs are located in the short arm of the human acrocentric chromosomes 13-15, 21 and 22 and they are related with the proliferative and metabolic cellular activities (2). The amount of proteins associated to NORs stained with the colloidal silver technique (AgNORs) reflects the degree of differentiation of the neoplasms (3).
AgNOR reaction was described first in 1975 by Goodpasture and Blomm (4). Afterwards, in 1986, Ploton demonstrated that this technique was useful to recognize cells in interphase stabilizating the reaction with formic acid (5). The quantity of AgNOR-associated proteins reflects the cellular differentiation degree and allows determining the proliferation and differentiation indexes of the transformed cells.
Exfoliative cytology is rarely used to evaluate the effect of tobacco smoking on the oral mucosa of the chronic smokers. This method can be made in the oral cavity since it is an easy, fast, simple and painless procedure. To date, only three studies were published reporting results on the AgNOR quantity in oral mucosal desquamative cells of chronic smokers (6-8). Any of the published studies included periodontally health patients. Results from the above mentioned studies (6-8) showed that tobacco burning products influence the proliferative capacity of the oral mucosal cells.
Periodontal disease is one of more frequent oral diseases in the world population and its main associated factor is poor oral cleaning and also, one of the associated risks for oral cancer is poor oral health too. In this fashion, people with good oral health procedures should present lower risk to develop oral cancer lesions. To date, no studies on AgNOR quantification in desquamated oral cells from periodontally health smokers in order to assess the risk to malignant transformation in epithelial cells from periodontally health chronic smokers (PHCS) compared with periodontally health non-smokers (PHNS) were published to date.
In view that studies on this matter are not published, the aim of this study was to quantify the AgNOR number in desquamative cells of two different sites of the oral mucosa of PHCS and PHNS as a marker of cellular proliferation using a Ploton’s modified technique was carried out.
MATERIAL AND METHODS
With a previously obtained written consent, the oral cavity of all PHCS attendants to the Preventive Tobacco Research Program located in the Faculty of Medicine, UNAM were chosen and a complete clinical oral examination including periodontal assessment was done. The research protocol was approved by the Ethics Committee of the Facultad de Medicina, UNAM. In all the PHCS selected patients, the oral examination was aimed to localize tobacco associated lesions. These patients were part of 2,452 volunteers participating in the above mentioned program. PHNS were selected from the students of our institution (Facultad de Odontología, UNAM. Mexico City, MEXICO). All the reviewed patients were among 30 and 55 years.
Periodontal health was considered when under clinical scrutiny no gingival inflammation was seen and periodontal probing was between 3-4 mm depth. All volunteers with antecedents of systemic diseases, previous periodontal treatment or under antibiotic therapy were excluded.
From the participants, 84 cytological smears were prepared. They were done scrapping the buccal mucosa and the dorsal surface of the tongue with a wooden tongue depressor with no previous rinsing; the obtained material was extended over a glass slide and fixed with 95% aqueous alcohol solution 4 smears each patient were stained with the Papanicolaou’s stain technique.
42 cytological smears were stained with a modified method to the Ploton’s technique of silver nitrate for AgNORs (5). Our modification was as follows: we used a 2% gelatin and 1% formic acid solution. Then, we mixed one part of this solution with two parts of a 50% silver nitrate aqueous solution. Afterwards, we immersed the slides in this mixture during 45 minutes at room temperature (20-25°C), solution was discarded.
AgNOR counting was done selecting several 100X random fields for each slide (not less than 100 cells per slide). The slides were observed in a Carl Zeiss bright field microscope with a 100X objective and immersion oil. We obtained mean, standard deviation and percentages. Data were analyzed by the Student T test, p<0.05 was considered statistically significant.
RESULTS
From the 2,452 volunteers, only 11 PHCS (0.45%) were found and 10 PHNS students from our institution fulfilling the above mentioned criteria were included.
In the microscopic review of the cytological smears stained with the Papanicolaou’s technique we observed numerous desquamated cells in almost all the lingual cases. In the smears from the buccal mucosa, desquamated cells were in lesser quantity. Despite some variations in the nuclear shape and size, no obvious signs of dysplasia were observed. Inflammatory infiltrate and colonies of microorganisms were also observed.
In the slides prepared with our modification to the Ploton’s technique, the usual black background was not observed. AgNORs were visualized as black dots within the cellular nucleus and variation in their size and quantity was observed. In the reviewed smears, AgNOR number varied from one to 11 per nucleus. We found that in PH smokers’ smears, AgNOR number varied from 3 to 11 (4.66±2.43). AgNOR number in cells recovered from the buccal mucosa was between 3 and 11 per nucleus and AgNOR number in lingual cell nuclei was between 2 and 8 (fig. 1). These figures were different to AgNOR counts found in squames recovered from PH non-smokers. In desquamated cells from both areas of PH non-smokers, range was among one and three AgNORs per nucleus (fig. 2) with a mean of 1.6 (SD±2.5). Differences in AgNOR number between both areas in PH smokers and AgNOR counts among PH smokers and PH non-smokers were statistically significant (p= 0.001 and p= 0.007 respectively).

Fig. 1:
Photomicrograph showing 2 or 3 AgNORs in a desquamated cell from non-smokers.
Modified Ploton’s technique. Scale Barr 1µ.
Fig. 2:
Several AgNORs can be seen in desquamative cells from smokers. Modified Ploton’s
technique. 100X.
DISCUSSION
Exfoliative cytology is widely used long time ago as a supporting tool to predict early changes (pre-neoplastic or neoplastic) in desquamative cells from the oral mucosa. It is well understood that exfoliative cytology never will substitute the biopsy and this procedure is widely used as a previous test in white lesions suspicious of malignancy. Even exfoliative cytology is a non-invasive and easy to achieve procedure and the silver staining technique allows the assessment of the cellular proliferative capacity. In our review of the literature, we found only three studies reporting on the use of these techniques for assessing the tobacco effect on the oral mucosa of chronic smokers (6-8).
Silver staining technique for AgNORs is used to distinguish among malignant and benign lesions (9), and it is considered an important tool for diagnosis in some neoplasms (10). do Carmo and Silva reported that AgNOR numbers are increased in cells with highly proliferative activity (10). Jaber and co-workers pointed out that tobacco and alcohol are important risk factors in lesions showing epithelial dysplasia (11).
Separate studies (6-8) reported AgNOR number differences in desquamated cells from smokers compared with AgNOR counts in non-smokers’ cells. In this work we found that AgNOR number in PHCS was 4.66 an in PHNS smokers it was 1.6. In this study and in the previously reported works (6-8), statistically significant differences among both groups were found. Our results showed that higher AgNOR number was found in PHCS compared with PHNS. These results suggest that despite the excellent oral health and cleaning procedures made by PHCS, they have a higher risk to develop pre-malignant lesions or even oral cancer, compared with PHNS. Also it should be pointed out that tobacco smokers are at major risk to develop premalignant oral lesions and even oral cancer compared with non-smokers.
Figures in our study and those previously reported (6-8) suggest that heat and the chemical products released during tobacco burn-up increase the proliferative capability of the oral mucosal epithelial cells. Also, our results showed that AgNOR counts in epithelial cells from the buccal and lingual mucosa in both, PHCS and PHNS are different. These results suggest that these differences in AgNOR counts from both areas should be taken in count when assessment of tobacco habit risk is evaluated.
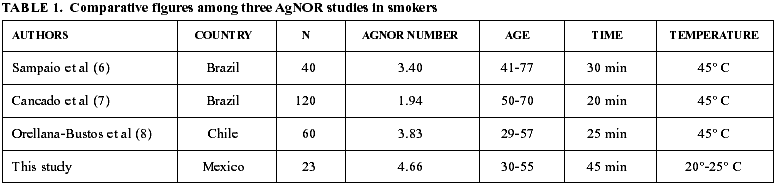
It has been suggested that the time employed in soaking the smears within the silver solution does not influence the AgNOR count and that differences in the previously reported figures (6-8) could be related to the age of the participants. This is because it has been described that oral mucosa during aging has a smaller proliferation activity (13). As it is shown in table 1, AgNOR counts in the previously reported studies (6-8) give the impression that this phenomenon is not influenced by the soaking time and these authors suggest that age of the patients has an effect on these figures. This finding is not supported by our results, since age of our population was among 30 and 55 years and the AgNOR counts reported here are higher than the previously reported studies (6-8,14). In this moment, we can not ascertain with confidence if differences in AgNOR counts reported in this study are related with ethnic factors or if the modified technique used in this study has a higher sensitivity.
The use of the method employed here shows several advantages; we did not use heat, this is clearly beneficial since smear processing is easier and burning of the sample during the staining procedure is prevented. Another advantage of our modification is that despite the participants did not rinse the oral cavity with any kind of solution, we did not detect the dark background commonly seen when the smears were stained with our modification to Ploton’s silver technique (5). We consider that for the above mentioned reasons, this simplification to the Ploton’s technique, it may be used as a primary test in underdeveloped countries and later, whenever possible, finer immunohistochemical and more expensive tests as antibody against Ki-67 nuclear antigen can be used to confirm if the risk is certain.
We like to point out that in the review of the studied slides, a cellular pattern suggesting dysplastic changes or malignant transformation was never found. The importance of the results obtained in this study is that a relationship among tobacco smoking and the increase of the proliferative activity of the oral mucosal epithelial cells exists. Also, it is important to indicate that our modification to the Ploton’s technique used in this study demonstrated to be highly efficient, effortless and easier to use.
The authors strongly recommend achieving more studies in this field using the simplified method we employed in this study.
REFERENCES
Andre K, Schraub S, Mercier M, Bontemps P. Role of alcohol and tobacco in the aetiology of head and neck cancer: a case-control study in de Doubs region in France. Oral Oncol Eur J Cancer 1995; 31B: 301-9.
Arden KC, Pathak S, Stettner S, Ritchie E. Differential silver-positive nucleolus organizer region activity in normal and malignant murine tissues. Cancer Genet Cytogenet 1989; 37: 55-60.
Hirsch SM, DuCanto J, Cardarelli DD, Hutchinson JC, Coon JS. Nucleolar organizer regions in squamous cell carcinoma of the head and neck. Laryngoscope 1992; 102: 39-44.
Goodpasture C, Bloom SE. Visualization of nucleolar organizer regions in mammalian chromosomes using silver staining. Chromosoma 1975; 53: 37-40.
Ploton D, Menager M, Jeannesson P, Himber G, Pigeon F, Adnet JJ. Improvement in the staining and the visualization of the argyrophilic proteins of the nucleolar organizer regions at the optical level. Histochem J 1986; 18: 5-14.
Sampaio H de C, Loyola AM, Gómez RS, Mesquita RA. AgNOR count in exfoliative cytology of normal buccal mucosa. Effect of smoking. Acta Cytol 1999; 43: 117-20.
Cancado RP, Yurgel LS, Sant’Anna-Filho M. Evaluation of nucleolar organizer region associated proteins in exfoliative cytology of normal buccal mucosa. Effect of smoking. Oral Oncol 2001; 37: 446-54.
Orellana-Bustos AI, Espinoza-Santander IL, Franco-Martinez ME, Lobos-Jaimes-Freyre N, Ortega-Pinto AV. Evaluation of keratinization and AgNORs count in exfoliative cytology of normal oral mucosa from smokers and non-smokers. Med Oral 2004; 9: 197-203.
Cardilho MR. AgNORs technique in fine needle aspiration cytology of salivary gland masses. Acta Cytol 1992; 21: 275-9.
Derenzi M. The AgNORs. Micron 2000; 31: 117-20.
Do Carmo MAV, Silva EC. Argyrophilic nucleolar organizer regions (AgNORs) in ameloblastomas and adenomatoid odontogenic tumours (AOTs). J Oral Pathol Med 1998; 27: 153-6.
Jaber MA, Porter SR, Gilthorpe MS, Bedi R, Scully C. Risk factors for oral epithelial dysplasia- the role of smoking and alcohol. Oral Oncol 1999, 35: 151-6.
Hill MW, Squier CA. Epithelial proliferation and turnover in oral epithelia and epidermis. En: Squier CA, Hill MW, eds. The Effect of Aging in Oral Mucosa and Skin. Boca Raton: CRC Press; 1994. p. 75-83.
Remmerbach TW, Weidenbach H, Muller C, Hemprich A, Pomjanski N, Buckstegge B, Bocking A. Diagnostic value of nucleolar organizer regions (AgNORs) in brush biopsies of suspicious lesions of the oral cavity. Anal Cell Pathol. 2003; 25: 139-46.