
Vol. 37, n.º 2, 2004
REVISTA
ESPAÑOLA DE
Vol. 37, n.º 2, 2004 |
Jesús Vera Álvarez, Miguel Marigil-Gómez, Manuel Abascal Agorreta, María Dolores García-Prats, Miguel Lacasa Laliena1, Miguel Piris-Pinilla2
Servicio de Anatomía Patológica y 1 Otorrinolaringología. Hospital General San Jorge. Huesca. 2 Centro Nacional de Investigaciones Oncológicas Carlos III. Madrid. jvera@hsjr.insalud.es
SUMMARY
A case of CD30 (Ki-1) anaplastic primary cutaneous large-cell lymphoma (CLCL) with histologic characteristics of epithelioid sarcoma is reported. CD30-positive anaplastic primary CLCL is a recently described clinicopathologic entity, recognized by immunohistochemical criteria. We describe a 74-year-old woman with an ulcerated tumour on her trunk, with 3 months evolution. The diagnosis was made on the basis of immunohistochemical findings. Systemic involvement was not detected. After a 6-year follow-up period, the patient is well, without concurrent extracutaneous disease. Clinical studies indicate a better prognosis of primary cutaneous CD30-positive large-cell lymphoma as compared with their nodal counterparts and with other CLCL. This case is in keeping with other reports of spontaneous regression and favourable outcome of this type of cutaneous lymphoma.
Keywords: large cell lymphoma, cutaneous lymphoma, CD30 antigen, epithelioid sarcoma.
RESUMEN
Presentamos un caso de linfoma primario cutáneo de células grandes CD30 (Ki-1) positivo con características histológicas que recuerdan a un sarcoma epitelioide. El linfoma primario cutáneo anaplásico de células grandes CD30 positivo es una entidad clinicopatológica recientemente descrita, reconocida por criterios inmunohistoquímicos. Describimos el caso de una mujer de 74 años de edad con un tumor ulcerado en el tronco, de 3 meses de evolución. El diagnóstico se hizo en base a los hallazgos inmunohistoquímicos. No se detectó afectación sistémica. La paciente está bien, sin enfermedad extracutánea concurrente, tras 6 años de seguimiento. Los estudios clínicos indican un mejor pronóstico en los linfomas cutáneos anaplásicos de células grandes CD30 positivos que en los linfomas anaplásicos de células grandes CD30 positivos nodales y que en los otros linfomas cutáneos anaplásicos de células grandes. Este es pues, un caso de regresión espontánea y seguimiento favorable en este tipo de linfomas cutáneos, que tiene unos rasgos morfológicos peculiares, como es su parecido morfológico con un sarcoma epitelioide.
Palabras clave: linfoma de células grandes, linfoma cutáneo, CD30, sarcoma epitelioide.
INTRODUCTION
CD30 primary CLCL represents a heterogeneous group of lymphoproliferative disorders that clinically originate in the skin and, after a variable period of time, may progress to involve lymph nodes, peripheral blood and/or visceral organs (1). A spectrum of CD30-positive primary cutaneous lymphoproliferative disorders has been identified in which spontaneous regression of skin lesions frequently occurs and includes lymphomatoid papulosis (LyP), regressing atypical histiocytosis (now included within the wider group of CD30-positive lymphomas) and CD30 (Ki-1)-positive large-cell lymphomas (2). The clinical and histological heterogeneity of these tumours and their confusing terminology have caused considerable confusion amongst dermatologists, haematologists and pathologists.
The identification of this entity is often difficult in the skin due to morphological features that overlap with other malignancies, such as malignant melanoma, poorly differentiated carcinoma and other large cell lymphomas. Furthermore, in some cases, the presence of granulomata generates a broad differential diagnosis that includes infectious and non-infectious causes.
CD30 primary CLCL is also known as «Ki-1 positive lymphoma». It was described in the skin by Kaudewitz and co-workers (3), and has been recognized as a neoplasm manifested as cutaneous nodules composed of lymphocytes with large, strikingly atypical nuclei. By definition, the neoplastic cells expressed Ki-1 (CD30).
CASE REPORT
A 74-year-old woman presented a 3-month history of a relatively painless cutaneous nodule localized on the trunk that enlarged progressively and became ulcerated. The lesion was 1.5 cm in diameter, firm, indurated and with a central ulceration. No other signs nor symptoms were expressed. There were neither palpable lymph nodes nor hepatosplenomegaly. Complete blood-cell count, serum biochemistry and immunoglobulins, bacterial and viral cultures, and Epstein-Barr virus antibody titers were either within normal limits or negative. Chest radiography and computed tomographic scans of the thorax and abdomen were normal and no additional therapy was required. There is no evidence of disease six years after surgery.
MATERIAL AND METHODS
Skin biopsy was fixed in 10% buffered formalin, embedded in paraffin, cut in 4 µm sections and stained with haematoxylin and eosin, periodic acid-Schiff (PAS), acid-fast and methenamine silver stains.
Sections were studied immunohistochemically with automated immunostaining on a Biotek Solutions Tech Mate (TechMate 500; Biotech Solutions, Dako, Glostrup, Denmark) and then incubated in a detection kit (CheMate, code K4001, Dako) according to the manufacturer’s instructions. Peroxidase activity was developed with 3-3’-diaminobenzidine (Sigma Chemical Co.) to obtain a brown end product. Heat-induced epitope retrieval was performed for all sections by means of a pressure cooker, as previously described (4). Representative sections were examined by using positive and negative controls. The following commercially available antibodies were employed: epithelial membrane antigen (EMA) (E 29, 1:100) (Biogenex, San Ramon, CA; USA), a low-molecular-weight cytokeratin marker (CAM 5.2) (1:100), CD15 (Leu M1) (MMA, 1:10) (Becton Dickinson, San Jose, CA, USA), vimentin (V9, 1:200), melanoma marker (HMB-45) (1:30) (Novocastra, Newcastle, UK), leukocyte common antigen (CD45) (2B11 and PD7/26, 1:200), CD3 (1:100), CD45RO (UCHL1, 1:100), CD20 (L26, 1:50), CD79a (JCB117, 1:50), CD30 (Ber-H2, 1:20), keratin 19 (RCK 108, 1:50), a macrophage marker (CD68) (KP1, 1:50), and Epstein-Barr virus (EBV) (CS 1-4) (Dako, Carpenteria, CA, USA).
RESULTS
Microscopically, the most striking feature was the presence in the reticular dermis and subcutaneous tissue of irregular nodules with central necrosis (fig. 1), surrounded mainly by large, polymorphic, highly atypical cells, with abundant amphophilic cytoplasm (fig. 2). The nuclei were vesicular and appeared variably round, oval, irregular o lobulated with coarse granular chromatin and one or more prominent nucleoli (fig. 1). Mitotic figures were frequent, and occasional multinucleate cells were present. A modest inflammatory background with eosinophils, neutrophils, scattered small lymphocytes and histiocytes were seen in the infiltrate. No acid-fast bacilli or fungal organisms were identified by acid-fast, PAS or Gomori methenamine silver stains.
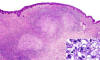
Fig. 1: Dermal infiltrate showing nodular
aggregates of tumour cells bordering a central area of necrosis.
(Haematoxylin-eosin stain x40). Inset shows a high power of the neoplastic cells
with abundant cytoplasm, irregular nuclei and prominent nucleoli.
(Haematoxylin-eosin stain x400).
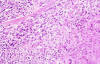
Fig. 2: Tumour cells surround a zone of
necrosis. (Haematoxylin-eosin stain x200).
Immunohistochemically, the large pleomorphic cells expressed the CD30 (Ki-1) antigen (fig. 3); were also positive for CD45 (fig. 4) and EMA, and negative for CD15, CD20, CD79a, CD3, CD45RO, CD68 antibodies as well as for cytokeratin (CAM 5.2), keratin 19, vimentin, HMB-45, and EBV.
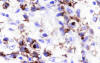
Fig. 3: Atypical cells showing membranous
staining and paranuclear dot-like reaction with CD30. (Ber H2 x400).
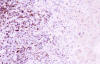
Fig. 4: Neoplastic cells stained with
CD45. (CD45 x200).
DISCUSSION
CLCL represent a heterogeneous group of malignant lymphomas that clinically originate in the skin and show considerable variation in clinical presentation, histological appearance, immunophenotype and prognosis.
Of particular importance has been the recognition that, in contrast to lymph node-derived lesions which disseminate widely, rapidly and have a poor prognosis, CD30 (Ki-1/Ber-H2)-positive anaplastic large-cell lymphoma arising primarily in the skin have, in general, a favourable outcome. As a consequence it has been suggested that CD30 expression is the single most important prognostic indicator for primary CLCL and that histological subtyping may be of less clinical significance (3,5). CD30-positive CLCL is a rare condition, occurring in all age groups. Although predominantly found in adults with a median age of 60 years, approximately 20% of the cases reported corresponded to patients under 20. A male preponderance is commonly documented. CLCL present most often as erythematous dome-shaped tumour nodules, either solitary or as a small cluster, and are usually confined to a single anatomical region. Occasionally papules, plaques and ulcerated lesions may be evident. The head, trunk, arms and legs are equally affected (3). Most cases arise de novo, but a few have been reported as engrafted on mycosis fungoides (MF) in Hodgkin’s disease or developing in HIV-infected patients (6).
CD30-positive primary cutaneous tumours have a survival rate of 75% while approximately 25% of the cases eventually develop nodal involvement. Poor prognostic indicators within the group include widespread skin lesions at presentation and high tissue eosinophilia (5). The prognosis was especially poor for patients with skin lesions in the context of a systemic CD30-positive lymphoma (2).
Immunophenotypically, CD30 primary CLCL have been of T-cell origin or, in some patients, of indeterminate (non-B/non-T cell) origin (5). The tumour cells are, by definition, CD30 (Ki-1)-positive. There is also consistent positivity for EMA and interleukin-2-receptor, particularly in the systemic cases. Ki-67 is usually strongly positive (7).
Primary cutaneous CD30-positive large-cell lymphoma must be differentiated from CD30-negative lymphoma since previous studies have suggested that patients with the latter have a much worse prognosis than patients with the former (3,5). Also, distinction must be made between cutaneous CD30-positive lymphomas that develop de novo in the skin and cutaneous CD30-positive lymphomas that develop in patients with MF or other types of cutaneous T-cell lymphomas. Patients from this last category generally have a poor prognosis (8).
The occurrence of spontaneous remission in almost 25% of the CD30-positive lymphomas suggests a relationship between these lymphomas and LyP, since it has a benign clinical course and falls in the histological spectrum of CD30-positive lymphoproliferative disorders. Therefore, clinical data instead of histological appearance are fundamental in making the diagnosis (9).
CD30 primary CLCL histologically resembling sarcoidosis (10), angiosarcoma (11) and infectious granuloma (12) have been reported. Our case histologically mimics an epithelioid sarcoma, containing nodular aggregates of anaplastic large cells with central necrosis. The differential diagnosis included high-grade non-Hodgkin’s lymphoma, Hodgkin disease, malignant melanoma, poorly differentiated carcinoma, epithelioid sarcoma and infectious processes with abscess formation.
Immunohistochemistry was helpful in making the diagnosis of anaplastic large-cell lymphoma; CD30 (Ki-1) and EMA were positive in the cytoplasm of tumour cells. The neoplastic cells did not react with cytokeratin (CAM 5.2), keratin 19, CD15 and HMB-45. This uniform absence of stain is valuable in excluding epithelioid sarcoma, anaplastic carcinoma, Hodgkin disease, or melanoma.
A recent study has shown that CD95 expression is preferentially expressed at high levels in all cutaneous CD30-positive lymphomas and suggests that CD95 may play a role in the regression of CD30-positive skin lesions (13).
The genetic alterations responsible for the development of cutaneous lymphoma are largely unknown. Recently, an allelic deletion at 9p21-22 in primary cutaneous CD30-positive large-cell lymphoma (14) has been found.
Recognition of these CD30-positive primary CLCL as a distinct type of lymphoma with a favourable prognosis is of great importance because it may prevent patients from undergoing unnecessary aggressive treatment. This makes the investigation of CD30 expression imperative, in all primary CLCL.
REFERENCES
Edelson RL. Cutaneous T-cell lymphoma: mycosis fungoides, Sèzary´s syndrome and other variants. J Am Acad Dermatol 1980; 2: 89-106.
Paulli M, Berti E, Rosso R, Boveri E, Kindl S, Klersy C, et al. CD30/Ki-1 positive lymphoproliferative disorders of the skin: Clinicopathologic correlation and statistical analysis of 86 cases: A multicentric study from the European Organization for Reseach and Treatment of Cancer Cutaneous Lymphoma Project Group. J Clin Oncol 1995; 13: 1343-54.
Kaudewitz P, Stein H, Dallenbach F, Eckert F, Bieber K, Burg G, et al. Primary and secondary Ki-1+ (CD30+) anaplastic large cell lymphomas. Morphologic, immunohistologic, and clinical characteristics. Am J Pathol 1989; 135: 359-67.
Miller RT, Estran C. Heat-induced epitope retrieval with a pressure cooker. Appl Immunohistochem 1995; 3: 190-3.
Beljaards RC, Kaudewitz P, Berti E, Gianotti R, Neumann C, Rosso R, et al. Primary cutaneous CD30-positive large cell lymphoma: Definition of a new type of cutaneous lymphoma with a favorable prognosis: An European multicenter study on 47 patients. Cancer 1993; 71: 2097-104.
Tirelli U, Vaccher E, Zanogel V, Talamini R, Bernardi D, Tavio M, et al. CD-30 (Ki-1)-positive anaplastic large-cell lymphomas in 13 patients with and 27 patients without human immunodeficiency virus infections. The first comparative clinicopathologic study from a single institution that also includes 80 patients with other human immunodeficiency virus-related systemic lymphomas. J Clin Oncol 1995; 13: 373-80.
Delsol G, Al Saati T, Gatter KC, Gerdes J, Schwarting R, Caveriviere P, et al. Coexpression of epithelial membrane antigen (EMA), Ki-1, and interleukin-2 receptor by anaplastic large cell lymphomas. Diagnostic value in so-called malignant histiocytosis. Am J Pathol 1998; 130: 59-70.
Camisa C, Helm TN, Sexton C, Tuthill R. Ki-1-positive anaplastic large cell lymphoma can mimic benign dermatoses. J Am Acad Dermatol 1993; 29: 696-700.
Willenze R, Kerl H, Sterry W, Berti E, Cerroni L, Chimenti S, et al. EORTC classification for primary cutaneous lymphomas: a proposal from the cutaneous lymphoma study group of the European Organization for Research and Treatment of Cancer. Blood 1997; 90: 354-71.
Boulier I, Cohen J, Kerdel FA. Cutaneous large-cell lymphoma histologically resembling sarcoidosis. J Am Acad Dermatol 1993; 2: 327-30.
Beylot-Barry M, Vergier B, Beylot C, Demascarel A, Doutre MS. Ki-1-positive large-cell cutaneous lymphoma mimicking a Stewart-Treves angiosarcoma. Dermatology 1995; 190: 77-82.
Montemarano AD, Rowe JE, Benson PM, Krishnan J. Ki-1 (CD-30)-positive anaplastic large cell lymphoma mimicking an infectious granuloma. Int J Dermatol 1995; 34: 790-3.
Paulli M, Berti E, Boveri E, Kindl S, Bonoldi E, Gambini C, et al. Cutaneous CD30+ lymphoproliferative disorders: Expression of bcl-2 and proteins of the tumour necrosis factor receptor superfamily. Hum Pathol 1998; 29: 1223-30.
Boni R, Xin H, Kamarashev J, Utzinger E, Dummer R, Kempf W, et al. Allelic deletion at 9p21-22 in primary cutaneous CD30(+) large cell lymphoma. J Invest Dermatol 2000; 115: 1104-7.